ENDOMETRIOSIS
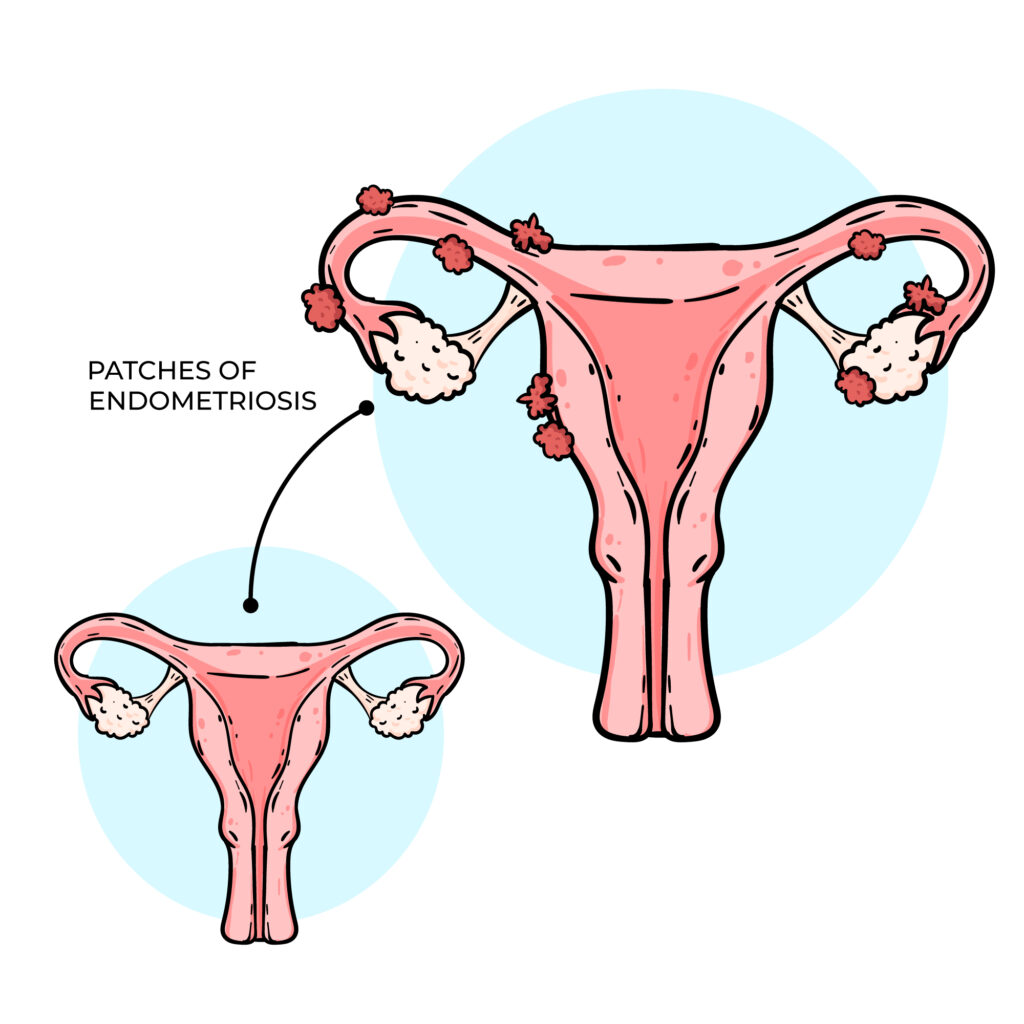
Endometriosis occurs when the tissue that normally lines the uterus and sheds during menstruation grows outside its usual place, often causing painful periods and affecting fertility. This tissue can grow within the womb’s lining and continue to spread as long as menstruation continues. Complete removal is usually not possible, as taking out too much can damage the uterus and affect the ability to carry a pregnancy, In severe cases, surgery may be done to remove some of the tissue, followed by hormone injections that stop menstruation for 3-6 months, allowing remaining tissue to shrink. However, a more effective approach may be to register with an IVF clinic, undergo endometriosis surgery, and then begin IVF treatment while menstruation is suppressed. This gives the body a better chance to conceive while preventing further tissue growth. It’s important to check and balance your AMH levels and hormones before surgery to improve your chances of success.
WHEN YOUR AMH VALUE IS ABOVE 3.5NG/ML AND YOU DON’T HAVE SIGNS AND SYMPTOM OF PCOS.
Anti-Müllerian Hormone (AMH) is a crucial marker used to assess a woman’s ovarian reserve in fertility evaluations. The typical reference range for AMH is approximately 1.0 to 3.5 ng/mL. Values below 1.0 ng/mL may indicate diminished ovarian reserve, while levels above 3.5 ng/mL can be suggestive of Polycystic Ovary Syndrome (PCOS). However, it’s important to note that not all women with elevated AMH levels present with the classical symptoms of PCOS, such as irregular menstrual cycles, acne, or anovulation. This condition is often referred to as “subclinical” or “silent PCOS”, where hormonal imbalance exists without obvious external signs. A high AMH level without symptoms may still affect egg quality and fertilization potential, potentially impacting conception outcomes. It is essential not to rely solely on regular menstruation or ovulation as indicators of reproductive health. If your AMH is above 3.5 ng/mL, or falls below 1.0 ng/mL, it is advisable to consult a fertility specialist for comprehensive evaluation and guidance on appropriate treatment or management strategies.
DIFFERENT TYPES OF FALLOPIAN TUBES DAMAGE.
Fallopian tube damage is a significant factor in female infertility. Common types of tubal pathology include: 1. Tubal Blockage: Where the fallopian tube is not patent (i.e., not open), preventing the passage of sperm or egg. 2. Hydrosalpinx: A condition where the tube becomes swollen and fluid-filled due to chronic inflammation, often impairing fertility. 3. Fimbrial Adhesions: Adhesions or scarring at the fimbrial end of the tube (the finger-like projections that help capture the egg from the ovary), which can block egg pick-up and transport. If one fallopian tube is damaged or surgically removed commonly after an ectopic pregnancy the other may still function and allow for natural conception. However, if one tube has hydrosalpinx, the toxic fluid it contains may backflow into the uterus and impair implantation, thereby affecting both natural and assisted conception (including IVF). A potential intervention is hydrotubation or tubal flushing, which aims to restore tubal patency. However, this procedure carries no guarantee of success and outcomes remain uncertain. After three months of any tubal treatment, a follow-up Hysterosalpingography (HSG) is recommended to reassess tubal status. If the tubes remain blocked or hydrosalpinx persists, in vitro fertilization (IVF) may be the most appropriate option for achieving pregnancy.
WHEN THERE IS NO GOOD RESULT FROM THE TREATMENT OF SEMEN PROBLEM.
There are instances where men undergo treatment for abnormal semen parameters, yet see little or no improvement in their semen analysis. For example, the sperm count may remain persistently low between 2 million and 10 million per milliliter or may even decline further, despite ongoing medication or therapy. In some cases, hormonal profiles and scrotal ultrasound results appear normal, yet sperm count, motility, and morphology fail to improve. One often overlooked cause is a blockage or dysfunction in the epididymis the coiled tube located at the back of the testicle where sperm is stored and matures. If this duct is obstructed or not functioning properly, sperm cannot pass through effectively, and medical treatments aimed at boosting sperm production may not yield results. In such cases, it is essential to consult a qualified fertility specialist or andrologist who can identify the underlying issue and recommend appropriate management options, which may include further diagnostic evaluation, surgical intervention, or assisted reproductive techniques such as IVF with ICSI.
SEVERE LOW SPERM COUNT AND AZOOSPERMIA
If you have been diagnosed with azoospermia (complete absence of sperm in the ejaculate) or severe oligospermia (sperm count less than 5 million/ml), it’s important to understand that the chances of spontaneous recovery with medication alone are often limited. Be cautious of anyone claiming to have a guaranteed cure. There are two main types of azoospermia: 1. Obstructive Azoospermia This occurs when sperm production in the testicles is normal, but a blockage prevents the sperm from reaching the ejaculate. Common causes include obstructions in the epididymis, vas deferens, or ejaculatory ducts, and may present as an epididymal cyst. These cases are often challenging and require evaluation by a urologist or male fertility specialist for possible surgical correction or assisted reproductive options like sperm retrieval. 2. Non-Obstructive Azoospermia This form results from impaired or absent sperm production by the testicles. In such cases, a scrotal ultrasound and hormonal profile (e.g., FSH, LH, testosterone) are essential to assess testicular function. Possible causes include undescended testes (cryptorchidism) or testicular failure due to heat damage or developmental issues. If the testes did not properly descend during childhood and remained inside the body beyond puberty, heat exposure may have irreversibly damaged sperm-producing cells. A quick self-check: normally, both testicles should be present in the scrotal sac, located below the penis. During cold temperatures, the scrotum may contract, but the testes should not retract entirely into the body. If you suspect you have a sperm production issue or have been diagnosed with azoospermia or severe oligospermia, consult a fertility scientist.
DON’T HAVE INTERCOURSE FROM A WEEK AFTER YOUR OVULATION.

For couples trying to conceive, timing of intercourse plays a significant role. Ovulation usually occurs around the mid-cycle, typically between day 10 and day 14 of a regular menstrual cycle. This is the most fertile window, and couples are generally advised to have intercourse during this period to maximize the chances of conception. After ovulation, the luteal phase begins. During this phase, the fertilized egg, if conception has occurred it travels to the uterus for implantation. At this point, excessive or poorly timed intercourse does not enhance conception and, in some cases, may interfere with implantation. Healthcare professionals often advise moderation after ovulation, allowing the body to support early implantation and stable hormone balance. Always consult your fertility specialist for personalized guidance, as recommendations may vary based on individual medical history and fertility treatment plans.
FIBROID COULD PREVENT YOU FROM GETTING PREGNANT.
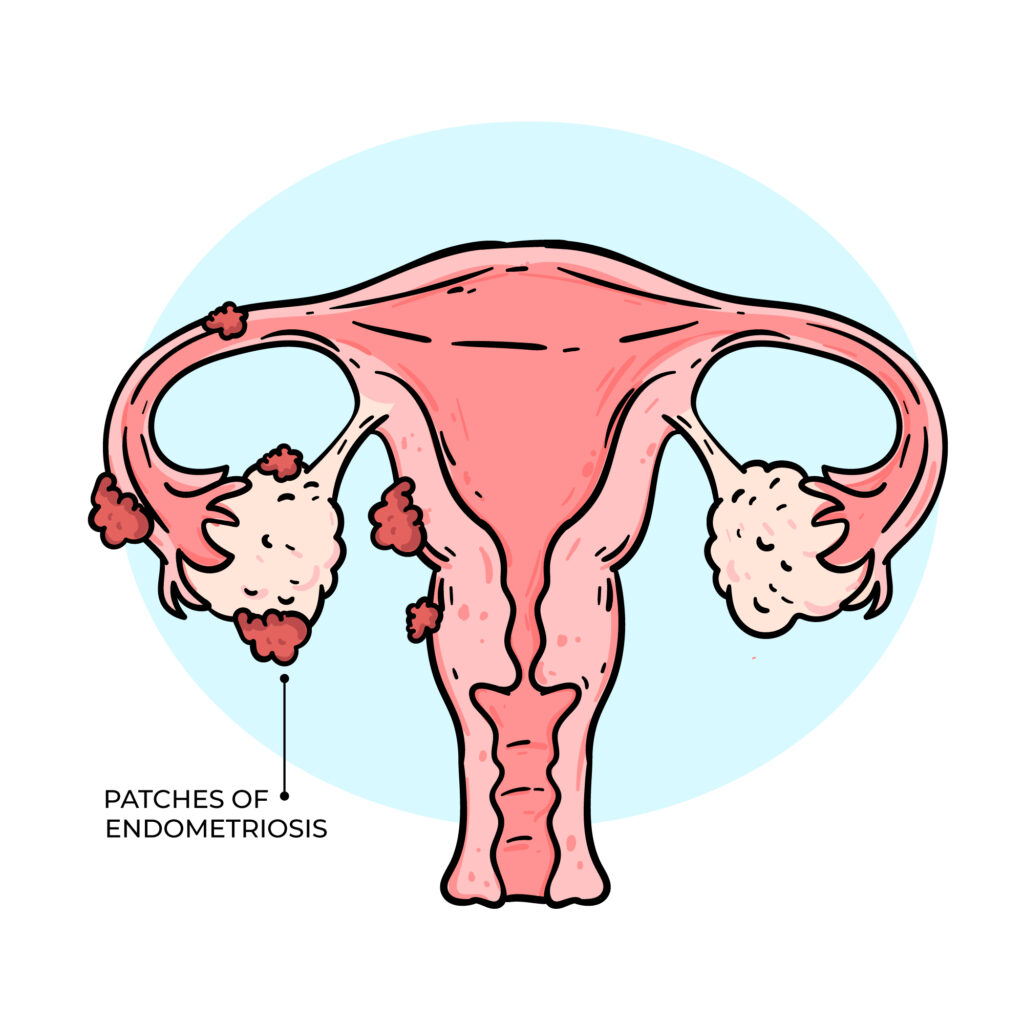
Uterine fibroids are noncancerous growths of the uterus that can affect a woman’s ability to conceive, depending on their size, number, and location. Certain types of fibroids, especially those that distort the uterine cavity (submucosal fibroids), may interfere with blood supply and nutrient flow to the endometrium, thereby affecting implantation and pregnancy. While some fibroids may not significantly impact fertility, others can pose challenges. The main ways fibroids may affect conception include: 1. Reduced implantation potential Fibroids that distort the uterine cavity can decrease the ability of the embryo to successfully implant, even when they are relatively small. 2. Hormonal influence Some fibroids may alter the local hormonal environment of the uterus, affecting fertility. Research suggests that fibroids can influence the balance of reproductive hormones, potentially reducing the likelihood of pregnancy. 3. Uterine contractility Normal uterine contractility gentle wave-like movements of the uterus helps facilitate embryo implantation and early pregnancy development. Fibroids can disrupt this process, making it more difficult for the uterus to support a pregnancy. For these reasons, fibroids should not be dismissed as harmless in women who are experiencing infertility or recurrent pregnancy loss. A thorough evaluation is recommended to determine whether fibroids may be contributing to fertility challenges and to guide appropriate management.
CAN HORMONAL TESTS BE DONE AT ANY TIME DURING THE MENSTRUAL CYCLE?

Traditionally, hormonal tests such as Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH) are performed on day 2 or day 3 of the menstrual cycle. The reason is that, at this stage, these hormones are usually at a stable baseline, making it possible to assess ovarian reserve more accurately. However, with the advancement of reproductive medicine, Anti-Müllerian Hormone (AMH) testing has become the gold standard for evaluating ovarian reserve. Unlike FSH and LH, AMH can be measured at any point in the menstrual cycle, as its levels remain relatively stable throughout. AMH provides a more sensitive and reliable indication of the remaining egg supply and overall ovarian function. It is important to understand that the menstrual cycle is divided into three phases: Follicular Phase (menstrual bleeding to ovulation) Ovulatory Phase (release of the egg) Luteal Phase (post-ovulation until the next cycle begins) A professional who understands these phases and the dynamics of reproductive hormones will be able to determine which tests are cycle-dependent (like FSH and LH) and which are cycle-independent (like AMH).
OVARIAN REJUVENATION

IMPORTANT INFORMATION FOR WOMEN WITH LOW OVARIAN RESERVE This is for women who have been diagnosed with low ovarian reserve, premature ovarian insufficiency, or chronic PCOS, and are exploring options to conceive using their own eggs. The desire to conceive with one’s own eggs is completely valid and deeply personal. However, when ovarian reserve is diminished or absent, options become more limited. One experimental approach currently being explored is known as ovarian rejuvenation. Ovarian rejuvenation refers to a research-based, experimental procedure aimed at stimulating or restoring ovarian function in women with low or absent egg reserves. It is hypothesized to potentially reactivate dormant or aging ovarian tissue, possibly leading to the production of viable eggs. However, it is important to note that this treatment is not yet proven, and its efficacy and safety have not been fully established in large-scale clinical studies. There are three proposed methods of ovarian rejuvenation: 1. Platelet-Rich Plasma (PRP) Therapy – Involves injecting PRP directly into the ovaries. It is thought to help stimulate follicle development and improve ovarian function. This is currently the most commonly used technique in countries like Nigeria. 2. Stem Cell Therapy – Involves the use of stem cells (often from the patient’s own bone marrow or adipose tissue) to potentially regenerate ovarian tissue. 3. In vitro Activation – This involves using certain agents or compounds to trigger dormant follicles to resume growth. Before considering any form of ovarian rejuvenation, it is essential to: Undergo a full reproductive hormone panel, including Anti-Müllerian Hormone (AMH) testing, to assess your current ovarian reserve. Consult with a board-certified fertility specialist Patients should be fully informed and cautious, as many clinics may offer these services without sufficient scientific backing or regulatory approval. Final Thoughts: While the idea of ovarian rejuvenation offers hope, it is important not to make decisions based on hype or pressure. Always seek evidence-based advice from qualified medical professionals, and remember that this approach remains investigational at this time.
Uterine Cramping After Ovulation

Experiencing uterine cramps after ovulation, either continuously until your next menstrual period or several days after ovulation, may be linked to different underlying causes. Two common possibilities include: 1. Incomplete Follicular Maturation or Follicular Persistence In some cases, ultrasound reports may show the presence of dominant follicles; however, some follicles may not have matured fully or may fail to rupture. When eggs do not reach maturity or are not released, these persistent follicles can lead to the formation of ovarian cysts. This process may trigger pelvic discomfort or cramping after ovulation. 2. Progesterone Insufficiency or Impaired Endometrial Response After ovulation, progesterone plays a key role in preparing the uterine lining (endometrium) for implantation. Low progesterone levels can cause uterine contractions or cramping due to inadequate support for implantation. In some cases, a woman may have normal progesterone levels, but the endometrium may not respond effectively to the hormone—a condition sometimes referred to as reduced endometrial receptivity. Why Evaluation is Important If you experience persistent cramping after ovulation, it is important to consult a qualified healthcare professional. Hormonal evaluation, pelvic ultrasound, and endometrial receptivity assessment may be required to identify the cause. Addressing hormonal imbalance, improving follicular development, and enhancing endometrial receptivity can increase the chances of successful implantation and pregnancy.
